In standard PCR, the enzyme, primers, other reagents, and templates are mixed at room temperature and left at this temperature before the first temperature cycle is initiated
1. Introduction
In standard PCR, the enzyme, primers, other reagents, and templates are mixed at room temperature and left at this temperature before the first temperature cycle is initiated ![]() )
)![]() )
)![]() )
)
2. Hot-Start
One way to minimize the primer-dimer formation ![]() )
)![]() )
)
3. Methods for Hot-Start
The simplest way to perform a Hot-Start is manually. This is done by heating the NucleoLink Strips in the thermal cycler to the denaturation temperature, removing the tape while the liquid is still hot, add the Taq-polymerase, and reclosing the Strips with new tape. This approach is acceptable as a first test for optimization, but only in research laboratories. Moreover, great care must be taken to avoid carry-over contamination ![]() )
)![]() )
)
3 a). Commercial approaches to Hot-Start
At present there are two commercial products available incorporating with a technique that uses inactivated Taq-polymerase:
The major drawback of these products is their relatively high price.
3 b). Wax overlay
A more low-cost approach is to employ wax instead of the oil overlay normally used to reduce evaporation (Wainwright and Seifert, 1993). The wax is added to the PCR mixture prior to addition of a compound required for the initiation of the reaction. These compounds may be: Primers, enzymes, or MgCl2. The mix is heated to melt the wax. After cooling, the wax makes a compact barrier. The missing reagent is added to the top of the wax, and as wax melts only at high temperatures, the two separate liquids will mix only after reaching the high temperature. The wax can be purchased as ready-to-use beads from a number of producers. The disadvantage of this method is the rather tedious heating and cooling required in the preparation of the PCR vessels. Furthermore, there have been reports of problems in getting the two separated liquids to mix through the wax.
4. Hot-Start in DIAPOPS
Since the DIAPOPS procedure behaves as a standard PCR, all the Hot-Start methods can theoretically be applied in DIAPOPS. Two of the methods have been tested by Nunc A/S research laboratory, namely the manual addition of enzyme to the NucleoLink Strips after heating, and the TaqStart™ antibody technique. Both methods enable the PCR and the solid phase detection to perform as expected, and no difference was seen compared with the test strips without Hot-Start. We believe that the expected positive difference will be seen in systems where the Hot-Start technique is required for the specific amplification to perform optimally. However, such a system has not yet been tested.
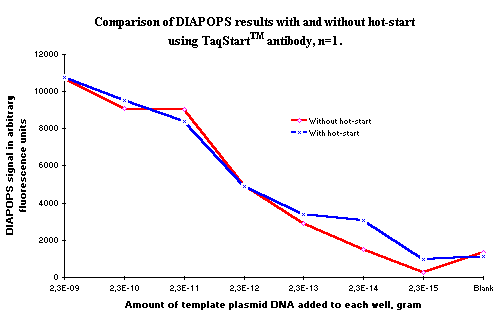 Figure 1: Showing DIAPOPS signals from two series of dilutions. Hot-Start was used in the experiment presented with a blue line, and standard PCR was used in the experiment presented with the red line. As observed, there is no significant difference in the DIAPOPS results in the system tested. |
DIAPOPS results from two dilution series are presented on Figure 1. PCR was performed with and without Hot-Start using the TaqStart™ antibody technique. The results showed that there was no difference in the DIAPOPS detection in this test system. This indicates that the test system does not gain from the Hot-Start technique, but it is also clear that it is possible to implement the Hot-Start technique in the DIAPOPS procedure without loosing performance. The difference between the curves consists of experimental variation, as only one NucleoLink Strip was included in each experiment.
5. Conclusion
The Hot-Start method can help to prevent unwanted hybridization in the start of the DNA amplification. There are several techniques for performing Hot-Start. Hot-Start can be used in DIAPOPS.