TechNote Volume 3 No. 17 - NucleoLink™ versus CovaLink™ Surfaces
When performing "ELISA like" assays with nucleic acids in MicroWell™ Plates, the adsorption of nucleic acid molecules onto the plastic surface is one factor which distinguishes success from failure.Nunc provides two types of surfaces for covalent immobilisation of nucleic acids: CovaLink and NucleoLink.
By a patented process the CovaLink surface is grafted covalently with a linker molecule ending in a secondary amino group. Nucleic acid molecules are bound at only the 5'-end to this linker molecule by carbodiimide condensation. The NucleoLink surface binds nucleic acids by carbodiimide condensation using similar procedure as with CovaLink ![]() )
)
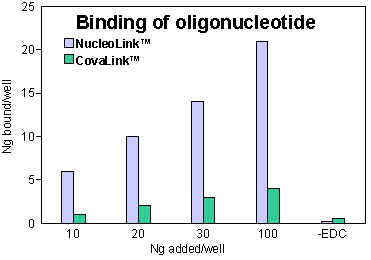 Figure 1: 10, 20, 30, and 100: The amount oligonucleotide (ng/well) present during coating.-EDC: Non-specific adsorption was tested by adding 100 ng oligonucleotide/well without EDC. |
Binding of oligonucleotide to NucleoLink and CovaLink
Binding of oligonucleotide to NucleoLink and CovaLinkVarious concentrations of 32P labelled oligonucleotide (0.133 ng/µl; 0.266 ng/µl; 0.4 ng/µl; 13.33 ng/µl) in 10 mM 1-methyl imidazole were pipetted into NucleoLink or CovaLink Strips, 75 µl/well. 25 µl freshly made 40 mM (EDC) in 10 mM 1-methyl imidazole was added to each well containing oligonucleotide. The CovaLink and NucleoLink Strips were then incubated at 50ºC for five hours. After incubation the strips were washed with 0.4 N NaOH, 0.25% Tween 20 using a Nunc Immuno™ Washer, Cat. No. 470173. The strips were washed three times, incubated at 50ºC for 15 minutes and finally washed three more times. The amount of covalently bound DNA was measured by liquid scintillation.
Conclusion:
20 to 50% of the oligonucleotide present during coating is bound to NucleoLink.
NucleoLink and CovaLink used for DIAPOPS
Six reaction mixtures were prepared exactly as described in TechNote Vol. 3 No. 16. To four of the mixtures 5 µl from four different known positive BLV (Bovine Leukemia virus) samples were added. To the two remaining 5 µl water were added. One half of each reaction mixture was added to a NucleoLink Strip, the other half to a CovaLink Strip. After amplification, hybridization and incubation with substrate, the fluorescence intensity was measured.
Conclusion:
The fluorescence intensifies obtained from the positive samples in NucleoLink Strips are approximately 4 times as high as the values from the same samples in CovaLink BreakApart Strips. Furthermore, the fluorescence intensifies obtained from negative samples in either strips are identical. Therefore, the use of NucleoLink for solid phase PCR assays greatly enhances the distinction between positive and negative samples. Based on experience the use of a primer longer than 25 bases for coating is recommended ![]() )
)
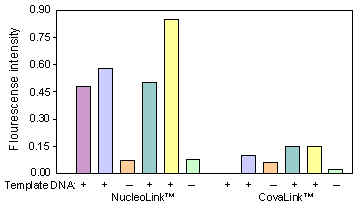 Figure 2: DIAPOPS ratios obtained in NucleoLink and CovaLink respectively. +: 5 µl template DNA added before amplification. -: 5 µl water added before amplification. |
NucleoLink and CovaLink used for DNA hybridization
Statens Serum Institut (SSI), Copenhagen, has developed a DNA hybridization assay for the detection of infectious organisms. The capture probe was bound as described above to CovaLink and NucleoLink. For the remainder of the assay, the CovaLink and NucleoLink Strips were used in parallel. After hybridization a chromogenic substrate was added. The colour development was measured on an ELISA plate reader.
Conclusion:
The results showed that by using NucleoLink, the amount of DNA needed for covalent immobilisation could be reduced by 90%. Furthermore, when using NucleoLink the time needed for substrate convention could be reduced by 50%.
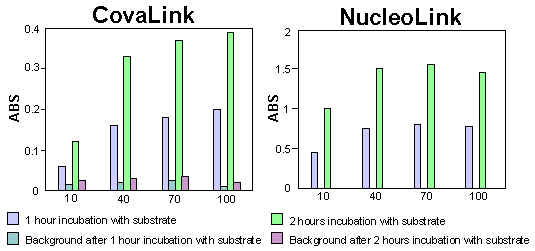 Figure 3 and 4: Uncoated strips were used for background measurements. Note the difference in y-axis. |