The alkaline phosphatase enzyme is used in the DIAPOPS procedure to generate a color or fluorescence signal from a substrate
1. Introduction
The alkaline phosphatase enzyme is used in the DIAPOPS procedure to generate a color or fluorescence signal from a substrate ![]() )
)![]() )
)![]() )
)
2. Data concerning Alkaline Phosphatase (AP)
The following data concerning AP is a summary of the material provided with the pure enzyme sold by Boehringer Mannheim (Phosphatase, alkaline (AP), EIA grade, Cat. No.: 567 752). This enzyme is used by DAKO A/S to prepare the streptavidin conjugated enzyme (DAKO, Streptavidin-AP, Cat. No. D0396), which is used in the DIAPOPS procedure.
The data describe the unconjugated enzyme. No data have been found on the optimal conditions of the conjugated enzyme. Although it is to be expected that the conjugated enzyme may yield slightly different optimum conditions in practical experiments, no difference has been observed in the numerous DIAPOPS experiments between the data given below and the performance of the streptavidin conjugated enzyme.
2 a). Nomenclature
Alkaline Phosphatase (AP): Orthophosphoric-monoester phosphohydrolase (alkaline optimum), EC 3.1.3.1.
2 b). Substrate specificity, relative rates and Km
Alkaline phosphatase (AP) hydrolyzes phosphate esters of primary and secondary alcohols, cyclic aliphatic alcohols, sugar alcohols, phenols, and amines. AP also hydrolyzes inorganic pyrophosphate (at a pH optimum of approx. 8) and 5'-terminal phosphates of single and double-stranded DNA or RNA. The enzyme will not hydrolyze phosphodiesters (R-O-PO2-O-R'; R, R': alkyl groups). Representative Michaelis constants (Km determined in 0.1 M Tris, pH 8.0 at 25ºC) include 4-nitrophenyl phosphate, 3.6 µM (relative rate = 1.0); pyrophosphate, 16 µM (rate = 0.9); AMP, 18 µM (rate = 1.2) ATP, 16 µM (rate = 0.9) and phosphoenolpyruvate, 5.5 µM (rate = 0.8).
The hydrolysis rate and substrate affinity of AP depend on a complex interrelation between the purity and the concentration of enzyme, buffer composition, ionic environment (ionic strength, solvents present), and pH. Specific use of AP requires careful, empirical optimization of the reaction conditions.
2 c). Enzyme structure and MW
AP is a dimer (MW = 140.000) of identical or nearly identical sub-units (MW = 69,000), each containing 2 molecules of Zn2+, one tightly bound and necessary for structural stability, the other loosely bound and required for catalytic activity. The active site contains a reactive serine. In some mammals (e.g. human), there are at least 3 distinguishable isoenzymes: the intestinal, the placental and the form found in bone/liver/kidney.
2 d). pH optimum
The optimal pH is between 8.0-10.5 (depending on substrate concentration). Figure 1 shows the dependence of pH of the activity under normal assay conditions such as those in the DIAPOPS procedure ![]() )
)![]() )
)
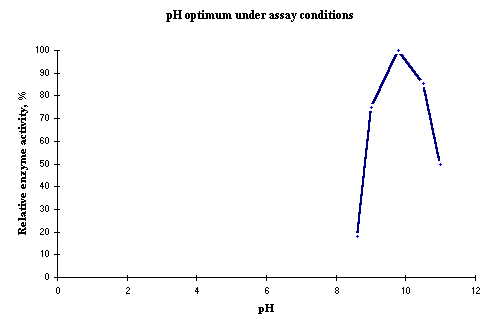 Figure 1: Relative AP enzyme activity as a function of the pH. |
2 e). Activators
Activators of AP are: Divalent metal ions (Mg2+, Co2+, Mn2+), amino alcohols (2-amino-2-methyl-1-propanol, diethanolamine), and TRIS buffer. In DIAPOPS, a diethanolamine buffer is used at pH 9.8 ![]() )
)
2 f). Inhibitors
Inorganic phosphate (Pi), monoethanolamine, Be2+, chelators of divalent metal ions (EDTA, oxalate, citrate, cysteine, histidine), acid or neutral pH, aromatic amino acids (Phe, Trp), L-homo-arginine, urea, iodoacetamide, and high levels of Zn2+ are inhibitory.
2 g). Temperature
The temperature dependence of the normal activity and the thermal stability of the enzyme after 10 minutes incubation in 3 M NaCl, pH 7.6, is presented in Figure 2.
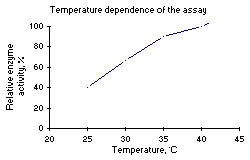 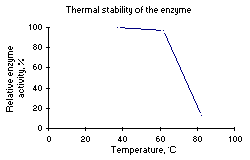 Figure 2: Left: Temperature dependence of the activity. Right: Thermal stability after 10 min. incubation. |
3. Incubation with streptavidin-alkaline phosphatase conjugate
Incubation with the streptavidin-enzyme conjugates is carried out at room temperature (RT) in standard ELISA procedures. In the DIAPOPS procedure ![]() )
)![]() )
)
4. Substrates tested in DIAPOPS
Only two substrates for AP have been tested in DIAPOPS, 4-MUP and pNPP. A comparison of the two tested substrates in a DIAPOPS assay is presented in Figure 6 ![]() )
)![]() )
)
4 a). 4-Methylumbelliferyl Phosphate (4-MUP)
4-MUP is a very sensitive substrate for AP, and it performs satisfactorily in DIAPOPS. The substrate becomes fluorescent when the phosphate group is removed by the hydrolyzation of AP. For detection of hydrolyzed 4-MUP, measure in a fluorescence plate reader. Use an excitation wavelength of 360 nm and an emission wavelength of 450 nm, even if the reaction has been stopped with K2HPO4 ![]() )
)![]() )
)
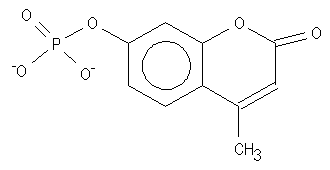 Figure 3: 4-MUP structure |
4 ai). Storage of the 4-MUP dilution
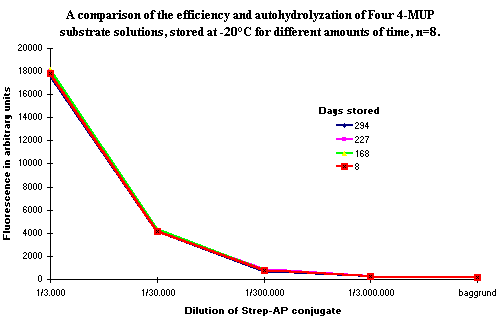 Figure 4: Showing the difference in fluorescence of four enzyme solutions stored at -20ºC for different amounts of time. The results show, that no decrease in efficiency, and no raised background signals from autohydrolysis are seen, even after a period of 10 months storage. |
A comparison was made of the ability to create a signal and of the background level from four different batches of 4-MUP ready-to-use substrate dilutions stored for up to 10 months at -20ºC ![]() )
)![]() )
)
The results show, that there is no difference in the ability of the four batches of 4-MUP to be hydrolyzed by the enzyme. Furthermore, no difference in background level is observed, indicating that no autohydrolysis has occured during the 10 months of storage at -20ºC.
4 b). para-Nitro Phenyl Phosphate (pNPP)
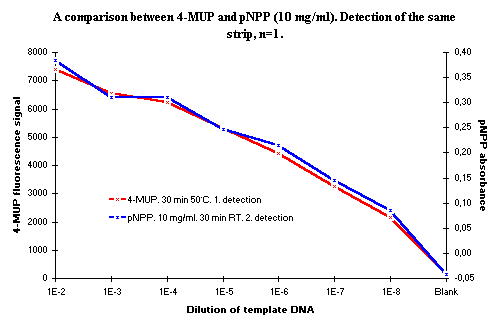 Figure 6: A comparison between substrates 4-MUP and pNPP. The same NucleoLink Strips were detected with rehybridization |
5. Conclusion
Alkaline phosphatase may be used in the DIAPOPS procedure with high performance. The incubation temperature of the Streptavidin-AP conjugate binding incubation should be controlled, and both 37ºC and 50ºC can be used. Two substrates for this enzyme have been tested in the DIAPOPS procedure, 4-MUP and pNPP. 4-MUP is the more sensitive substrate, but, if used in a concentration of 10 mg/ml, pNPP can give results as quickly and with the same performance as that of 4-MUP. Ready-to-use dilutions of 4-MUP in diethanolamine can be stored for up to 10 months at -20ºC without losing the ability to be hydrolyzed and without creating a higher background signal.