In the DIAPOPS system the solid phase product is detected by hybridization with a labelled probe. After detection of the label on the probe, the solid phase product remains covalently bound to the NucleoLink surface, unless acid is added
Rehybridization to the solid phase PCR product
1. Introduction
In the DIAPOPS system the solid phase product is detected by hybridization with a labelled probe. After detection of the label on the probe, the solid phase product remains covalently bound to the NucleoLink surface, unless acid is added ![]() )
)![]() )
)
The hybridization probe can be removed from the solid phase product after hybridization. This is performed by denaturation using the same procedure as for denaturing the double-stranded solid phase PCR product after the PCR. After this denaturation, the NucleoLink strips with the solid phase product can be stored and reanalyzed with a new hybridization, i.e. a rehybridization.
2. Reasons for rehybridization
There are two major reasons, why a new detection using rehybridization can be interesting.
2. a) Optimization of the hybridization conditions
In the optimization of individual DIAPOPS assays the hybridization step ![]() )
)![]() )
)
2. b) Verification of results
A new analysis of the solid phase bound product can be helpful in a later reanalysis when confirming a very important result or testing a new probe. The NucleoLink Strips with the immobilized amplicons can be stored empty after denaturation for long time at 4ºC ![]() )
)
3. Rehybridizations
It is possible to detect the solid phase product numerous times with rehybridization. Figure 1 shows results from three subsequent hybridization detections of the same NucleoLink Strips. The hybridizations are numbered 3, 4, and 5. The first two hybridizations were performed with lower probe concentrations and were used in another experiment, which is presented elsewhere ![]() )
)
As observed from the data in Figure 1, it is possible to detect the solid phase product at least five times, and to lose only a small amount of signal. The third hybridization, which yields the highest level of signal, is at a level which is normal for detection of this system in DIAPOPS. The reason to the decrease in the signals, is most likely a modest decay of the solid phase PCR product ![]() )
)
4. Variations in rehybridization data
In most systems, the first hybridization yields the highest signals. The subsequent hybridization detections lose signal, but only moderately. However, it in one system it was observed that the first hybridization did not yield the highest signal. In Figure 2, results are presented from four identical hybridization detections of a solid phase product from this system. As seen, the second and third hybridization show higher results than the first and fourth hybridization. The first hybridization shows an unexpected decrease in signal from the two samples with the highest template concentration. This may be due to problems in the wash after PCR ![]() )
)![]() )
)
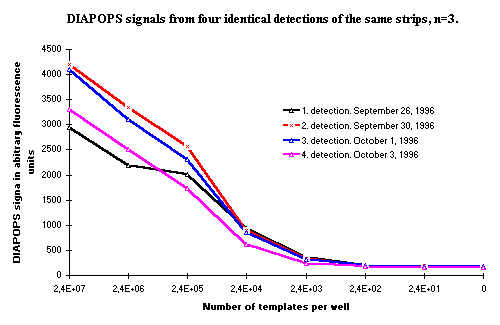 Figure 2: DIAPOPS signals from four different, identical hybridizations of the same solid phase product. The first hybridization detection is lower than expected in the two samples with the highest template concentration, which may be due to problems in the wash after PCR. This is only seen in some systems, but the rehybridization results clearly show that if such a problem is present, it can be analyzed using rehybridization. |
5. Conclusion
It is possible to detect the solid phase PCR products five times and possible more using new hybridizations - rehybridizations. The reason for this can be either to optimize the hybridization conditions or to verify an old result. It has been observed that one system showed a lower level of signals in the first hybridization, but the subsequent hybridizations showed increasing signals. This is most likely due to insufficient denaturation of the solid phase product in the first denaturation after the DNA amplification.