Heat stability of the covalently bound solid phase primer
- Introduction
- Analysis of the heat-stability of the covalently bound solid phase primer
- Conclusion
1. Introduction
NucleoLink Strips are heated to 94-97ºC during the thermal cycling required for PCR (  ). If the PCR program maintains the strips at high temperature for 1 min. and runs 40 cycles, the total time at high temperature is 40 min. However, this does not include the time it takes for the thermal cycler to reach this temperature, which can be 10 sec above 90ºC for each cycle. This time at an elevated temperature and the fact that some applications use more than 40 cycles requires an analysis of the stability of the covalent binding of the primers for over 1 hour at a high temperature.
). If the PCR program maintains the strips at high temperature for 1 min. and runs 40 cycles, the total time at high temperature is 40 min. However, this does not include the time it takes for the thermal cycler to reach this temperature, which can be 10 sec above 90ºC for each cycle. This time at an elevated temperature and the fact that some applications use more than 40 cycles requires an analysis of the stability of the covalent binding of the primers for over 1 hour at a high temperature.
2. Analysis of the heat-stability of the covalently bound solid phase primer
The temperature stability of the covalently bound solid phase primer on the surface of the NucleoLink Strips was tested at three temperatures: 94ºC, 97ºC, and 100ºC, respectively. One hundred microlitres TRIS buffer, at the same concentration as used in PCR, was added to all the coated wells before heat treatment. The primers remaining after heat treatment were detected using hybridization to ensure that the detection results was from DNA which could be used for hybridization, and therefore also for PCR (  ). The result of the experiment is presented in Figure 1. As observed, approximately 30% of the covalently bound solid phase primers are removed or inactivated at 94ºC. At 97ºC and 100ºC approximately 40% of the primers are removed or inactivated, leaving approximately 60% to be detected by hybridization and presumably also to be active in DIAPOPS (
). The result of the experiment is presented in Figure 1. As observed, approximately 30% of the covalently bound solid phase primers are removed or inactivated at 94ºC. At 97ºC and 100ºC approximately 40% of the primers are removed or inactivated, leaving approximately 60% to be detected by hybridization and presumably also to be active in DIAPOPS (  ). There appears to be no difference in the decrease in signal between 97ºC and 100ºC.
). There appears to be no difference in the decrease in signal between 97ºC and 100ºC.
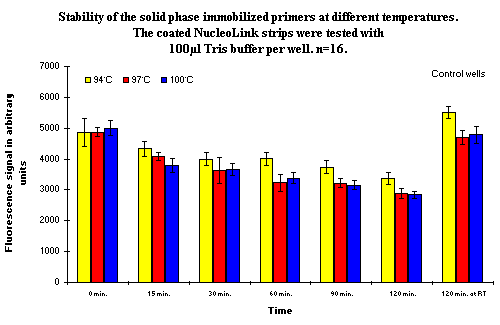
Figure 1: Illustrating the temperature stability of the covalently bound solid phase primer as a function of time at the temperature selected. The amount of covalently bound solid phase primers remaining after heat treatment was detected by hybridization (  ). The last measurement shows that the primers are stable in the liquid at room temperature and the decrease in signal is due solely to the temperature. The data from the experiment at 94ºC has been multiplied with a factor of 1.8 in order to be comparable with data from experiments performed at the other temperatures. The factor was calculated by comparing the binding rates in wells that was not heated. ). The last measurement shows that the primers are stable in the liquid at room temperature and the decrease in signal is due solely to the temperature. The data from the experiment at 94ºC has been multiplied with a factor of 1.8 in order to be comparable with data from experiments performed at the other temperatures. The factor was calculated by comparing the binding rates in wells that was not heated. |
3. Conclusion
As many as 70% of the primers are active after two hours at 94ºC, which is the normal denaturing temperature in PCR. At higher temperatures, more primers will be inactivated, and after 2 hours at 97ºC or at 100ºC, approximately 60% of the primers remain active.
![]() )
)![]() )
)![]() )
)