A case story of a background problem
- Introduction
- Set-up of the experiment
- Data
- Conclusion
1. Introduction
At the University of Gothenburg, Department of Clinical Immunology, Sweden, Liselott Svensson and Esbjörn Telemo (  ) were testing the DIAPOPS technique in NucleoLink Strips (
) were testing the DIAPOPS technique in NucleoLink Strips (  ). However, they were getting very high background signals (
). However, they were getting very high background signals (  ). Although positive signals were sometimes seen, they were only from samples with a very high template concentration; all other signals were masked by the high background signal. Preliminary experiments had shown that the source of background problems did not originate from only one source. The high background was even present in freshly coated NucleoLink Strips which had not previously been exposed to amplification or template. Blocking with BR (
). Although positive signals were sometimes seen, they were only from samples with a very high template concentration; all other signals were masked by the high background signal. Preliminary experiments had shown that the source of background problems did not originate from only one source. The high background was even present in freshly coated NucleoLink Strips which had not previously been exposed to amplification or template. Blocking with BR (  ) did not lower the background signals as previously seen (
) did not lower the background signals as previously seen (  ). In order to analyze the origin of this problem, the research laboratory at Nunc A/S and Liselott Svensson set up and conducted an experiment where the reagents from both laboratories were compared, and different solid phase primers (
). In order to analyze the origin of this problem, the research laboratory at Nunc A/S and Liselott Svensson set up and conducted an experiment where the reagents from both laboratories were compared, and different solid phase primers (  ) were analyzed.
) were analyzed.
2. Set-up of the experiment
In order to exactly pinpoint the location of the background, the DIAPOPS detection procedure (  ) was carried out in NucleoLink Strips coated with solid phase primers. In this way the analysis was only a detection of the background in empty coated wells. Reagents from the laboratory of Liselott Svensson and Esbjörn Telemo were compared to the reagents from the research laboratory at Nunc A/S.
) was carried out in NucleoLink Strips coated with solid phase primers. In this way the analysis was only a detection of the background in empty coated wells. Reagents from the laboratory of Liselott Svensson and Esbjörn Telemo were compared to the reagents from the research laboratory at Nunc A/S.
In the detection procedure (  ) each reagent was systematically left out to see when the background disappeared. In addition to this, two different syntheses of the same solid phase primer sequence (solid phase primer No. 1 and No. 2) from two different producers were tested as well as uncoated NucleoLink Strips. The solid phase primer (solid phase primer No. 1) from the laboratory of Liselott Svensson and Esbjörn Telemo was suspected of causing an increased background signal, as had previously been observed with another solid phase primer (
) each reagent was systematically left out to see when the background disappeared. In addition to this, two different syntheses of the same solid phase primer sequence (solid phase primer No. 1 and No. 2) from two different producers were tested as well as uncoated NucleoLink Strips. The solid phase primer (solid phase primer No. 1) from the laboratory of Liselott Svensson and Esbjörn Telemo was suspected of causing an increased background signal, as had previously been observed with another solid phase primer (  ). The solid phase primer used by the research laboratory at Nunc A/S (solid phase primer No. 2) had previously been seen to give a low background. Three different coatings from three different days were tested with this solid phase primer (solid phase primer No. 2).
). The solid phase primer used by the research laboratory at Nunc A/S (solid phase primer No. 2) had previously been seen to give a low background. Three different coatings from three different days were tested with this solid phase primer (solid phase primer No. 2).
3. Data
Each reagent was sequentially left out of the detection procedure (  ) in the experiment. The results are presented in Figures 1 and 2. Figure 1 shows the results from the detection procedure carried out in NucleoLink Strips with the reagents from the laboratory of Liselott Svensson and Esbjörn Telemo.
) in the experiment. The results are presented in Figures 1 and 2. Figure 1 shows the results from the detection procedure carried out in NucleoLink Strips with the reagents from the laboratory of Liselott Svensson and Esbjörn Telemo.
It is obvious that solid phase primer No. 1 yields a higher background compared to solid phase primer No. 2, which yields the same background signal as the uncoated Strip. The background does not decrease when the probe is left out, but disappears after the conjugate has been left out. This indicates that indeed there were two causes of the increased background signals, both a non-specific binding of the biotin recognizing conjugate from producer No. 1 (ExtrAvidin®-Alkaline Phosphatase, Sigma, Product No.: E 2636) to the NucleoLink Strips, and the even higher amount of conjugate binding to solid phase primer No. 1.
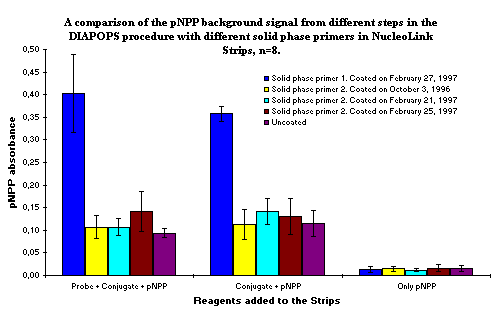
Figure 1: Results of the background signal in the DIAPOPS detection procedure carried out in NucleoLink Strips only coated with solid phase primers. In the detection step the probe, conjugate, and substrate was consecutively left out. The substrate was pNPP, and the biotin recognizing alkaline phosphatase conjugate (in short called "conjugate") was from producer No. 1. (ExtrAvidin®-Alkaline Phosphatase, Sigma, Product No.: E 2636) The reagents were from the laboratory of Liselott Svensson and Esbjörn Telemo. It is obvious that the conjugate attaches unspecifically, even to the uncoated Strips. It is also obvious that solid phase primer No. 1 binds a larger amount of conjugate than does the other solid phase primer. |
NucleoLink Strips identical to the ones tested in Figure 1 were tested in the same set-up with reagents from the Nunc A/S research laboratory where a different biotin recognizing conjugate was used (  ). The results showed that solid phase primer No. 1 did bind the streptavidin alkaline phosphatase conjugate unspecifically. The conjugate did not bind unspecifically to the Strips coated with solid phase primer No. 2 or to the uncoated Strips. This conjugate was from producer No. 2 (
). The results showed that solid phase primer No. 1 did bind the streptavidin alkaline phosphatase conjugate unspecifically. The conjugate did not bind unspecifically to the Strips coated with solid phase primer No. 2 or to the uncoated Strips. This conjugate was from producer No. 2 (  ).
).
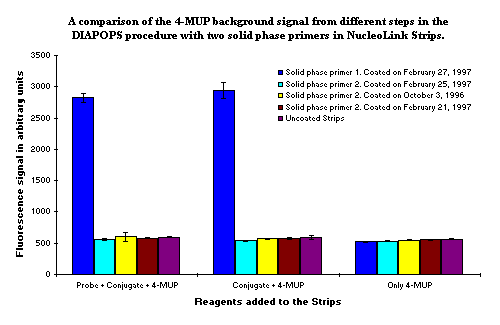
Figure 2: Results of the background signal in the DIAPOPS detection procedure carried out in NucleoLink Strips only coated with solid phase primers. In the detection step the probe, conjugate, and substrate was consecutively left out. The reagents were from the Nunc A/S research laboratory, where a different biotin recognizing conjugate was used. The substrate was 4-MUP, and the streptavidin-alkaline phosphatase conjugate (in short called "conjugate") was from producer No. 2 (  ). The conjugate only attached unspecifically to solid phase primer No. 1, but does not give any further background problems as did the conjugate from producer No. 1. ). The conjugate only attached unspecifically to solid phase primer No. 1, but does not give any further background problems as did the conjugate from producer No. 1. |
4. Conclusion
The background problem was found to originate from both the solid phase primer No. 1 and the biotin recognizing alkaline phosphatase conjugate from producer No. 1. The method of comparing different solid phase primers with an uncoated Strip and sequentially leaving out each reagent in the DIAPOPS detection procedure showed exactly where the origin of the background problem was. Furthermore, the comparison to an identical experiment with other reagents and another solid phase primer of the same sequence showed that the problem could be solved by purchasing new reagents and a new solid phase primer from other sources.
![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)
![]() )
)![]() )
)