Raised background signals in DIAPOPS
1. Introduction
Raised background signals in DIAPOPS ![]() )
)![]() )
)![]() )
)![]() )
)
2. Non-specific binding to the solid phase primer of the streptavidin conjugate
The reasons for the high background signals observed from the system in question were examined by analyzing each step in the DIAPOPS ![]() )
)![]() )
)![]() )
)
Most primers yielded a background of approximately the same level as the uncoated NucleoLink Strips. However, some primers bound the streptavidin conjugate and yielded high background signals. A very high background signal was observed with primer No. 8.
3. Blocking with BSA
In the experiment presented in Figure 1, pre-blocking with BSA as well as addition of 1 mg/ml BSA to the conjugate incubation buffer was examined to ascertain whether this would prevent the high background. Surprisingly, the result was the reverse, as shown in Figure 1 ![]() )
)
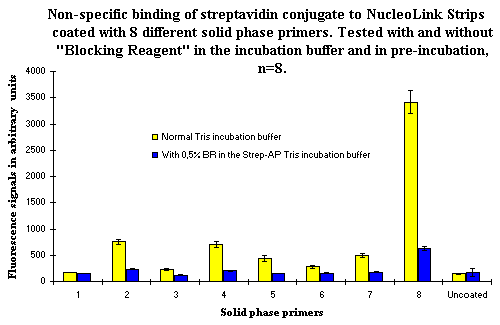 Figure 2: Non-specific binding of streptavidin conjugate to NucleoLink Strips coated with 8 different solid phase primers (also used in Figure 1). Non-specific binding was tested with and without 0,5% BR in pre-blocking and incubation buffer. Without blocking, most primers yielded slightly higher background signals than the uncoated NucleoLink wells, but some primers showed a higher non-specific affinity for streptavidin conjugate and yielded high background signals. The use of BR in pre-blocking and incubation buffer decreased the background level substantially. Only solid phase primer No. 8 yielded an elevated background signal after blocking with BR. |
In our search for a blocking agent that could reduce the non-specific background, we tested 'Blocking Reagent' (BR) from Boehringer & Mannheim. Results from this test are presented in Figure 2. The use of BR in pre-blocking and incubation buffer decreased the level background of background signals substantially. Only solid phase primer No. 8 still yielded an moderately elevated background signal after blocking with BR. It was obvious that inclusion of BR in buffers in the incubation step decreased the background level efficiently. However, this blocking with BR is not allways efficient. With one specific solid phase primer yielding a high background signal as a cause of non-specific binding of the streptavidin conjugate, blocking with BR did not solve the problem ![]() )
)
Following these experiments, we examined different buffers to determine in which buffers inclusion of BR was important. These results (data not shown) indicated that BR is only necessary in the incubation buffer, and this is now incorporated in the standard DIAPOPS procedure ![]() )
)
Further tests were performed to identify the buffer in which the addition of BSA causes an increased background signal. These results indicated (data not shown) that the background was only elevated if BSA was added to the incubation buffer. When BSA was used in the pre-blocking step, no increased background level was seen. No other blocking substance has been tested so far, but reagents similar to BR from Boehringer & Mannheim will most likely have the same blocking effect.
5. A new solid phase primer with the same sequence
From the data shown in Figures 1 and 2, it is obvious that the non-specific binding of the streptavidin conjugate is closely associated with each specific solid phase primer. As previously stated, the same pattern of increased background signals was seen in several experiments. The only apparent difference between the solid phase primers was the DNA sequences. To examine whether the non-specific binding of the streptavidin conjugate was DNA sequence specific, a new syntheses of primer No. 8 from Figure 1 and 2 was purchased. This new syntheses was from a different manufacturer than the first syntheses. As shown in Figure 3, this new primer syntheses did not bind the streptavidin conjugate non-specifically. The conclusion is that the non-specific streptavidin conjugate binding is not of DNA sequence specific origin.
It may be concluded that non-specific binding of the streptavidin conjugate is derived from unknown residues left over from the synthesis of the primer. This residue may be biotin. If a trace of biotin has been incorporated into the sequence of the solid phase primer during synthesis, this may explain the background.
The new solid phase primer No. 8 was HPLC purified, whereas none of the solid phase primers presented in Figures 1 and 2 were purified in any way. It has not yet been established whether purified solid phase primers may yield non-specific binding of the streptavidin conjugate and show increased background signals, but none of the purified primers tested so far has been observed to do this. For this reason, it is recommended that the solid phase primer is purified with e.g. HPLC before use. However, if the increased streptavidin conjugate binding of the solid phase primer is caused by erroneously incorporated biotin into the primer sequence, a purification will not solve the problem. In this case only a new synthesis of the primer sequence may resolve the background signal.
No problems have been observed with other primers from the manufacturer which synthesized the original primer No. 8 presented in Figure 1. From the data presented here it cannot be concluded that oligonucleotides from one source may be inferior to oligonucleotides from another source. The solid phase primer described elsewhere ![]() )
)
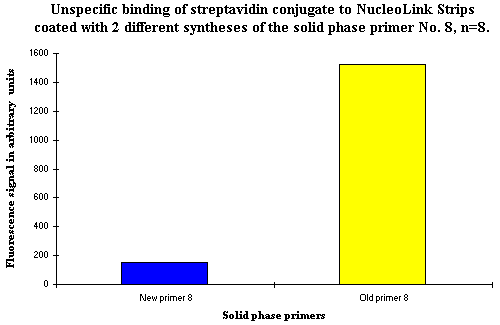 Figure 3: Illustrates the non-specific binding of the streptavidin conjugate to coated NucleoLink Strips as a function of two different syntheses of the same solid phase primer sequence. The primer sequence is the sequence also tested as primer No. 8 in Figures 1 and 2. |
6. Conclusion
The non-specific binding of the streptavidin conjugate is caused by some residue left over from the synthesis of the solid phase primer. This binding varies between different primer syntheses and is not correlated to the DNA sequence. The only way to solve the problem is to acquire a new synthesis of the solid phase primer or, possibly, to purify the problematic oligonucleotide before covalent binding to the NucleoLink Strips. Solid phase primers purified after synthesis have not been found to cause non-specific binding of the streptavidin conjugate.