Results from stability studies on the mix of primers and EDC before addition to the NucleoLink Strips
- Introduction
- Stability analysis of the primer and EDC mix
- Conclusion
1. Introduction
An EDC activated intermediate is unstable in aqueous solutions (  ). As a consequence, in the first DIAPOPS procedure EDC was not mixed with the solid phase primer before addition to the NucleoLink wells (
). As a consequence, in the first DIAPOPS procedure EDC was not mixed with the solid phase primer before addition to the NucleoLink wells (  ). In this initial procedure the solid phase primer was first added (100 ng in 75 µl) followed by EDC (25 µl of 40 mM EDC). This approach presents several problems:
). In this initial procedure the solid phase primer was first added (100 ng in 75 µl) followed by EDC (25 µl of 40 mM EDC). This approach presents several problems:
- A double workload, as it is necessary to add liquid twice to the same well.
- The risk of adding EDC twice or not at all as it is not clearly visible to which wells EDC has been added (
 ).
).
2. Stability analysis of the primer and EDC mix
In the DIAPOPS procedure the EDC and the phosphorylated solid phase primer are dissolved in a 1-methylimidazole buffer at pH 7.0 (  ). This buffer stabilizes the reaction (
). This buffer stabilizes the reaction (  ). It would solve the problems mentioned above, if the phosphorylated primer and EDC could be mixed before adding it to the wells in the NucleoLink Strips. An experiment was performed to analyze the consequence of this procedure on the binding efficiency. In this experiment the mix of primers and EDC in 1-methylimidazole buffer was left on the laboratory bench for different periods of time after mixing before it was adding it to the wells in the NucleoLink Strips. The resulting amount of bound solid phase primer was detected using hybridization with a biotinylated probe (
). It would solve the problems mentioned above, if the phosphorylated primer and EDC could be mixed before adding it to the wells in the NucleoLink Strips. An experiment was performed to analyze the consequence of this procedure on the binding efficiency. In this experiment the mix of primers and EDC in 1-methylimidazole buffer was left on the laboratory bench for different periods of time after mixing before it was adding it to the wells in the NucleoLink Strips. The resulting amount of bound solid phase primer was detected using hybridization with a biotinylated probe (  ).
).
As illustrated in Figure 1, the results indicate that there is no decrease in the signals, even when the mix of EDC and primers has been left overnight at room temperature. This demonstrates that it is possible to mix the EDC and solid phase primer before adding the mix to the wells of the NucleoLink Strips, a useful point when DIAPOPS is used as a routine method. This step is now used in the DIAPOPS procedure (  ).
).
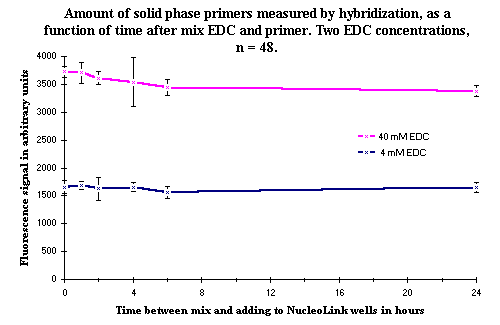 Figure 1: Shows the number of solid phase primers measured by hybridization as a function of the time between the mixing of the solid phase primers and the EDC and addition to the NucleoLink wells. The signal does not decrease significantly, even after 24 hours, which demonstrates that mixing the two components before the addition to the wells of the NucleoLink Strips does not constitute a problem. Figure 1: Shows the number of solid phase primers measured by hybridization as a function of the time between the mixing of the solid phase primers and the EDC and addition to the NucleoLink wells. The signal does not decrease significantly, even after 24 hours, which demonstrates that mixing the two components before the addition to the wells of the NucleoLink Strips does not constitute a problem. |
This alteration of the procedure also solves another problem: It is difficult to determine whether or not EDC has been added to the wells of the NucleoLink Strips (  ). If the person adding the liquid is interrupted during this process, there is a possibility of either not adding any EDC at all or adding twice the amount of EDC. Both possibilities will completely invalidate the DIAPOPS analysis (
). If the person adding the liquid is interrupted during this process, there is a possibility of either not adding any EDC at all or adding twice the amount of EDC. Both possibilities will completely invalidate the DIAPOPS analysis (  ). It is obvious that in the absence of EDC no primer will be bound to the solid phase. However, the addition of twice the normal amount of EDC will also reduce the number of solid phase primers substantially (
). It is obvious that in the absence of EDC no primer will be bound to the solid phase. However, the addition of twice the normal amount of EDC will also reduce the number of solid phase primers substantially (  ) and therefore the DIAPOPS result will be very poor. This problem can be avoided by mixing the phosphorylated solid phase primer and the EDC before adding the mix to the wells in the NucleoLink Strips.
) and therefore the DIAPOPS result will be very poor. This problem can be avoided by mixing the phosphorylated solid phase primer and the EDC before adding the mix to the wells in the NucleoLink Strips.
3. Conclusion
It is possible to mix the primer and the EDC in 1-methylimidazole buffer before adding the mix to the wells in the NucleoLink Strips. This approach will control the concentration of EDC and reduce the workload.
![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)![]() )
) Figure 1: Shows the number of solid phase primers measured by hybridization as a function of the time between the mixing of the solid phase primers and the EDC and addition to the NucleoLink wells. The signal does not decrease significantly, even after 24 hours, which demonstrates that mixing the two components before the addition to the wells of the NucleoLink Strips does not constitute a problem.
Figure 1: Shows the number of solid phase primers measured by hybridization as a function of the time between the mixing of the solid phase primers and the EDC and addition to the NucleoLink wells. The signal does not decrease significantly, even after 24 hours, which demonstrates that mixing the two components before the addition to the wells of the NucleoLink Strips does not constitute a problem. ![]() )
)![]() )
)![]() )
)