In DIAPOPS
General handling of NucleoLink Strips in DIAPOPS
1. Introduction
In DIAPOPS ![]() )
)![]() )
)
2. The term: Wash three times ![]()
This term means that the wells must be filled with washing liquid or washing buffer and emptied. This step should be repeated three times. After the last wash, the wells should be completely empty ![]() )
)![]() )
)
3. Volume of washing liquid
It is recommended that the volume of the washing buffer should exceed the volume of the incubation liquid. A volume of 100 µl is added to each well in the covalent binding of the solid phase primer at the beginning of the DIAPOPS procedure ![]() )
)
4. Tools for washing
When using a normal multichannel pipette ![]() )
)![]() )
)
5. Emptying the NucleoLink Strips
When washing the NucleoLink strips with a Nunc-Immuno Wash, the strips are emptied by suction between each wash. However, when the wells are washed, they are not completely empty as neither a pipette nor a wash can achieve this. Therefore, it is necessary to completely empty the wells after the washing by hitting them against a folded towel several times with the opening facing the towel. During this procedure the last remaining liquid is efficiently removed. In order to assure that the wells are as empty as possible the hitting should be rather vigorous. Since the Strips are locked very securely in the frame, this hitting should not dislodge the Strips from the frame.
6. The NucleoLink frames
The red NucleoLink frame locks on to every well in each NucleoLink Strip ![]() )
)![]() )
)
7. Sealing of NucleoLink Strips during DIAPOPS and storage
During thermal cycling the NucleoLink Strips are sealed with the heat-stable Tape 8 (Nunc Tape 8, Cat. No. 249719) to avoid evaporation. It is very important to assure that all wells are total closed when sealing the NucleoLink Strips with Tape 8. Tape 8 is designed to cover only one Strip. This is important, as the Strips are separately inserted to the thermal cycler ![]() )
)
As an alternative, it is possible to use the GeNunc Tape 48 (Nunc GeNunc Tape 48, Cat. No. 232700), which is also heat-resistant. However, use of the GeNunc Tape 48 demands some practice in inserting and removing the Strips from the thermal cycler. The Nunc Sealing Tape (Cat. No. 236366), which is the other tape used in the DIAPOPS procedure ![]() )
)![]() )
)![]() )
)
7 a). Evaporation during incubation
It is necessary to seal the NucleoLink Strips with tape. If the Strips are only closed with a lid, up to 10% evaporation will occur even for a short incubation period of one hour, as indicated in Figure 1. Closure with a lid (Nunc Lid for MicroWell® Plates, Cat. No. 263339) can only be used for a very short incubation e.g. 30 minutes as used during substrate incubation ![]() )
)
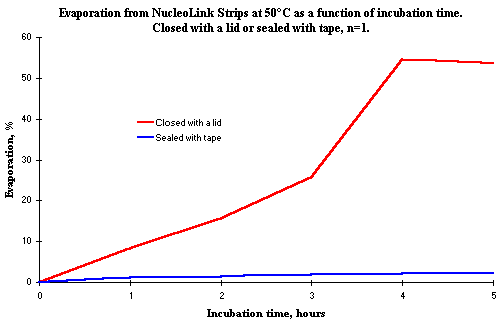 Figure 1: Illustrating the degree of evaporation in percent from NucleoLink Strips incubated at 50ºC, sealed either with Nunc Sealing Tape or only with a lid. A volume of 50 µl was initially added to the wells, and the evaporation was measured as the weight loss after incubation. The incubation was carried out in a incubator with a ventilator to ensure a uniform temperature. This explains why the evaporation is so high. It can be concluded that it is necessary to seal the Strips with tape, even if the incubation is only for one hour. The final measurement at 5 hours was made on Strips, which were not placed at the same site in the incubator as the other Strips, which explains the decrease in the rate of evaporation at this time. Evaporation is very dependent upon the airflow, which is not identical in different parts of the heating incubator. |
7 b). Sealing during storage
The NucleoLink strips can be stored at 4ºC after coating ![]() )
)![]() )
)![]() )
)![]() )
)![]() )
)
8. Removal of tape from NucleoLink Strips
To avoid tearing the NucleoLink Strips from the frame, when the tape is removed from the Strips after thermal cycling ![]() )
)![]() )
)
9. Inserting and removing NucleoLink Strips from thermal cyclers
The NucleoLink Strips are designed to fit into standard 0.2 ml thermal cycler blocks perfectly ![]() )
)![]() )
)![]() )
)
After insertion of the NucleoLink Strips into the thermal cycler, the heated lid is closed. It is very important to assure that the lid is firmly closed and that the pressure is applied. Applying a firm and even pressure during thermal cycling prevents evaporation from the NucleoLink Strips.
9 a) Use of GeNunc Tape 48 during thermal cycling
For an experienced user it is possible to seal the NucleoLink Strips with thermostable Tape 48 (Cat. No. 232700) before inserting them into the thermal cycler. As the red NucleoLink frame is not heat-stable, it must be removed before inserting the Strips into the thermal cycler. Great care must be exercised when removing the Strips from the thermal block after thermal cycling. Since the wells fit into the block perfectly, the Strips are firmly attached to the block after thermal cycling, and there is a risk of breaking the Strips. For this reason the removal of the strips must be performed carefully in order not to break the Strips. This can be accomplished by means of a standard angle pincer ![]() )
)![]() )
)
9 b) Spacer Plate
A silicone Spacer Plate is placed on top of the NucleoLink Strips during thermal cycling to assure that the heated lid provides an evenly distributed pressure to the Strips ![]() )
)
During thermal cycling, a small amount of glue from the tape will melt and adhere to the spacer plate. After prolonged use of the same spacer plate, the side facing the NucleoLink Strips will get sticky and may adhere to the Strips after thermal cycling. This can cause difficulties when the Spacer Plate is removed from the Strips, as the NucleoLink Strips will be lifted with the Spacer plate. Furthermore, if the Spacer Plate is turned so the side with the glue is facing the heated lid, the Spacer Plate may attach to the lid. The old Spacer Plate may remain attached to the heated lid. This may cause evaporation from a number of the wells during the next DIAPOPS analysis, if a new Spacer Plate is placed on the top of the NucleoLink Strips and the two Spacer Plates now used may create an uneven pressure on the Strips.
To remove the residual glue, the Spacer Plate should be regularly cleaned with ethanol.
10. ELISA readers
As the last step in the DIAPOPS procedure ![]() )
)![]() )
)
11. Can oil be used if the thermal cycler does not have a heated lid?
If the thermal cycler does not have a heated lid, or the lid is without pressure, it is possible to add one or two drops of oil to the NucleoLink wells after addition of the PCR mix. This will not affect the DIAPOPS results, if the oil is completely removed.
After thermal cycling, the liquid PCR mix is removed. If analysis of the liquid is not planned, the liquid and the oil are removed and discarded, as usual, and the wells are washed as described in the DIAPOPS procedure ![]() )
)![]() )
)
12. Conclusion
The NucleoLink Strips are handled much like MicroWell® plates during normal ELISA. However, the washing step must be carefully conducted, as the sensitivity of DIAPOPS is very high ![]() )
)