A number of different labels can be used with the probes in the hybridization detection step (
Probe concentration and probe labelling in DIAPOPS
1. Introduction
A number of different labels can be used with the probes in the hybridization detection step ( ![]() ) in the DIAPOPS (
) in the DIAPOPS ( ![]() ) technique. The following labels have been tested, in the Nunc A/S Research Laboratory:
) technique. The following labels have been tested, in the Nunc A/S Research Laboratory:
Radioactively labelled probes and probes labelled with Europium have been tested by external laboratories. Detailed results from these investigations are not presented, but results from the experiments were successful.
2. Biotinylated probes
A biotinylated probe has been used in most of the systems tested with DIAPOPS. An optimization of the probe concentration was carried out to determine a concentration giving the highest possible signal. The optimization was not made to find the lowest concentration yielding a detectable signal in order to minimize the costs. Furthermore, the lowest concentration yielding a sufficient, detectable signal is presumably different from one probe sequence to another and must be optimized for each individual system. The concentration of 50 nM biotinylated probe, suggested here ( ![]() ), has been tested with many different probe sequences, and was found to give good, high signals in all DIAPOPS systems tested (
), has been tested with many different probe sequences, and was found to give good, high signals in all DIAPOPS systems tested ( ![]() ).
).
It is possible to use concentrations lower than 50 nM, but the results will not show the same dynamic difference between samples with different initial template concentrations, and the limit of detection can also be affected negatively. Figure 1 shows data from three different concentrations of biotinylated probes. The results clearly show the difference in both the dynamic range and the limit of detection between the three probe concentrations.
The Strip analyzed in Figure 1, was subsequently rehybridized with a probe concentration of 50 nM. This is described elsewhere ( ![]() ).
).
3. AP labelled probe
The probe used for hybridization detection of the solid phase product in the DIAPOPS can be directly labelled with an enzyme, e.g. alkaline phosphatase (AP). This labelling is more expensive than labelling with biotin, and for this reason it is feasible only if probe concentrations lower than 50 nM also are usable. Figure 2 shows results from a DIAPOPS experiment using three different concentrations of AP labelled probe. The results are from different amplifications, and not from rehybridizations ( ![]() ) to the same amplicons, as are the results in Figure 1. The results presented in the Figures 1-3 are all from experiments using 4-MUP (
) to the same amplicons, as are the results in Figure 1. The results presented in the Figures 1-3 are all from experiments using 4-MUP ( ![]() ) as substrate.
) as substrate.
As can be seen in Figure 2, a concentration of 50 nM gave the best signal in DIAPOPS, and should therefore be selected. However, as mentioned above, the price for this probe is very high, and at Nunc A/S research laboratory we used a concentration of 5 nm with the probe directly labelled with alkaline phosphatase, which was acceptable, although the dynamic range was lower than with 50 nM.
3 a). Overnight hybridization
Hybridization can be performed overnight in the hybridization detection of the solid phase amplicons ( ![]() ). If overnight hybridization is carried out with a biotinylated probe, the results are identical to the results from a 1-hour hybridization with a biotinylated probe (data not shown). If the probe is directly labelled with alkaline phosphatase, the longer hybridization time gives lower signals due to enzyme instability. In Figure 3, overnight hybridization is compared with a hybridization carried out for one hour with different concentrations of the alkaline phosphatase labelled probe. The comparison was performed with two different hybridization buffers.
). If overnight hybridization is carried out with a biotinylated probe, the results are identical to the results from a 1-hour hybridization with a biotinylated probe (data not shown). If the probe is directly labelled with alkaline phosphatase, the longer hybridization time gives lower signals due to enzyme instability. In Figure 3, overnight hybridization is compared with a hybridization carried out for one hour with different concentrations of the alkaline phosphatase labelled probe. The comparison was performed with two different hybridization buffers.
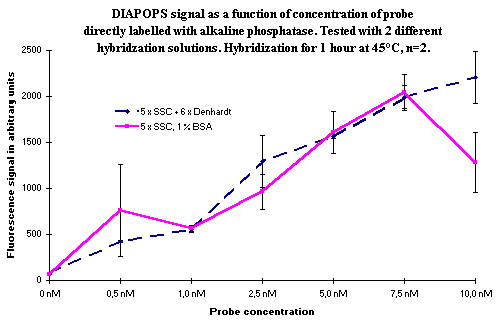 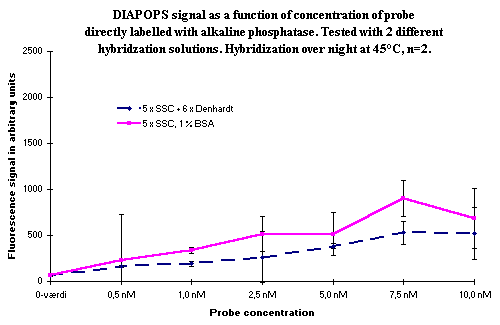 Figure 3: Comparison of hybridization for one hour (top) with overnight hybridization (bottom). Variation in hybridization solution and in concentration of the alkaline phosphatase labelled probe. No significant difference between the hybridization solutions was seen, although it appeared as though the mix without Denhardt's solution was somewhat better in the overnight incubation experiment. The increase in signal as a function of probe concentration is obvious, and it is also clear that an overnight incubation is not a good choice if the hybridization probe is directly labelled with alkaline phosphatase. |
As seen from the results in Figures 1-3, there is no significant difference in signals from the biotinylated probe and the probe directly labelled with alkaline phosphatase when the same concentration is used. This indicates that a high percentage of the biotinylated probes are recognized by the streptavidin alkaline phosphatase conjugate. Consequently, no gain in performance is seen when using the more expensive directly labelled probes. The advantage of the directly labelled probe is a faster assay. The conjugate binding step can be omitted, and thus about one hour of incubation and handling can be avoided.
4. Probe labelled with fluorescent tags
Two different labels have been tested: Fluorescein and Texas Red. If detection by hybridization can be successfully carried out with these labels, both the conjugate binding step and the enzyme reaction step can be omitted from the procedure, therefore time is saved. However, it was not possible to obtain useful signals with these fluorescent probes, because the concentration of the solid phase product is lower than the lowest concentration needed to give a signal with these fluorescent probes.
5. Conclusion
The optimal concentration to use with a biotinylated detection probe is 50 nM. It is possible to use a lower concentration, but only with loss of dynamic range. If a probe directly labelled with an enzyme is used, a concentration of 50 nM raises the price of the assay substantially. It is possible to use the lower concentration of 5 nM, but only with a decreased dynamic range. It is not feasible to use overnight hybridization with directly enzyme labelled probes, due to a degradation of the enzyme.
Two probes directly labelled with fluorescent tags has been tested. The fluorescent tags was; Fluorescein and Texas red. Neither of the probes could be detected after hybridization, and could therefore not be used for detection of the solid phase product. The reason is most likely to be the relatively low concentration of the solid phase product on the surface.